Like much of our society, the American university is increasingly scrutinized through an economic lens. The value of academic research is largely determined by its commercial potential or fundraising capacity. Much has been written about the corrosive effects of this point of view on the humanities, but it has transformed academic science and engineering as well. As Benjamin Ginsberg describes in his book The Fall of the Faculty (2011), universities have been flooded with administrators who view “faculty research mainly as a source of revenue” and “research projects as instruments that generate income.” Yet even the most application-driven universities have always aimed for something beyond enriching their endowment. MIT, for example, declares in its mission statement that it is “committed to generating, disseminating, and preserving knowledge, and to working with others to bring this knowledge to bear on the world’s great challenges,” doing it all “for the betterment of humankind.”
The CRISPR-Cas patent case brings out the ethical, economic, and conceptual problems with biomedical patents.
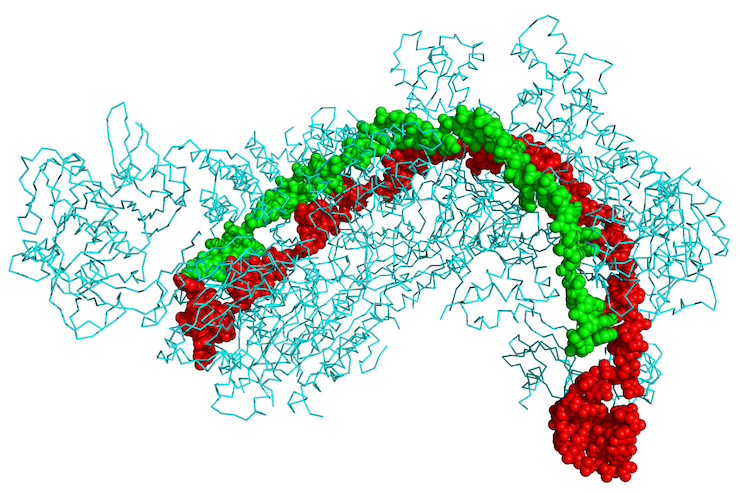
The recent discovery that the CRISPR-Cas system could be used for genome editing illustrates the tension between the corporatization of the university and the pursuit of basic, publicly funded research. Bacteria use CRISPR-Cas to attack the DNA of invading viruses. The workings of this natural defense mechanism were elucidated through basic research carried out mostly within universities. By hijacking and recombining its bacterial parts—a flexible kind of engineering that is the hallmark of molecular biology—researchers have shown how CRISPR-Cas can be used to edit the human genome, or any genome, in principle. The technique has been hailed as revolutionary, and there are high hopes for its applications in editing out genetic mutations that cause disease. The journal Science even dubbed the excitement the “CRISPR craze”: nearly 600 scientific papers were published on the subject in 2014 alone.
Alongside the excitement, a patent battle is raging between the University of California, Berkeley and the Broad Institute of MIT and Harvard. MIT’s Technology Review has called the legal dispute a “winner-take-all match” with “billion-dollar implications,” as the contenders all have stakes in startup companies based on the technique. The Broad team (in particular, the scientist Feng Zhang) was granted the first patent in 2014, but the Berkeley group (led by Jennifer Doudna) filed paperwork contesting the decision.
But the questions are more fundamental than who will collect the money. Do molecular entities have inventors, and can they be owned? Is it ethical for academic scientists to sell ownership or otherwise regulate access to new techniques? And, if it is acceptable to sell this sort of biological discovery, what are the implications for progress in medicine and science—and for the public that funds this work?
• • •
Before molecular biology took off in 1970s, the prevailing attitude toward patenting had been set by the Supreme Court in Funk Brothers Seed Co. vs. Kalo Inoculant Co. (1948), which ruled that living organisms extracted from nature cannot be patented. The Court held that “the qualities of these bacteria, like the heat of the sun, electricity, or the qualities of metals, are part of the storehouse of knowledge of all men” and are “reserved exclusively to none.” One who “discovers a hitherto unknown phenomenon of nature has no claim to a monopoly of it,” the justices concluded.
The rise of molecular biology complicated the notion of a “phenomenon of nature” by enabling parts of living systems to be recombined, using tools that are themselves parts of nature.
The rise of molecular biology complicated the notion of a “phenomenon of nature” by enabling parts of living systems to be recombined, using tools that are themselves parts of nature. Thus the question became at what point the reshuffling of natural parts makes a non-natural whole. The Court took up this issue in 1980, ruling in Diamond vs. Chakrabarty that living organisms modified by genetic engineering—in this case, bacteria modified to break down crude oil—could be patented. The Court reasoned that genetically modified bacteria are obtained by “nonnaturally occurring manufacture” and are therefore “a product of human ingenuity.” In the same year, the first of Stanford University’s Boyer-Cohen patents was approved for a method that used microbial cells to produce recombinant DNA, which is spliced together from different sources. The ability to create new DNA and use it to modify organisms would soon open the door to many more patents.
Shortly after the Diamond ruling, Congress passed the Bayh-Dole Act, which intensified the push for patents in academia by allowing universities to claim ownership of inventions based on publicly funded research. Technology license and transfer offices rapidly proliferated in universities, and academic molecular biology became a hunting ground for patent hawks. As Robert Cook-Deegan wrote in The Gene Wars (1994), a “virulent pest, the lawyer” invaded molecular biology, and as a result “DNA and the technologies to manipulate and analyze it became new frontiers for patent and copyright law.” Columbia University’s Axel patents on co-transformation, a method for introducing recombinant DNA into cells, were granted in 1983. Throughout the 1990s, scientists also rushed to patent genes and gene variants associated with disease. Francis Collins, now director of the National Institutes of Health (NIH), and his University of Michigan colleagues patented the cystic fibrosis gene discovered in the late 1980s. In the late ’90s, Myriad Genetics, cofounded by a University of Utah researcher, patented two genes associated with breast cancer. Craig Venter, then at the NIH, and colleagues attempted to patent genetic sequences derived from RNA, and Eric Lander, now director of the Broad Institute, and colleagues at MIT patented certain disease-associated gene variants.
Whether genes can be patented became the subject of intense debate in the 1990s during the race to sequence the human genome.
Whether genes can be patented became the subject of intense debate in the 1990s during the race to sequence the human genome. The public, NIH-backed sequencing effort, led by Collins and Lander, argued strongly against gene patenting. The question was eventually put to the Supreme Court in a case involving Myriad in 2013. In an amicus brief, Lander echoed the logic of Funk Brothers, arguing that genes shouldn’t be patentable because they are “products of nature.” The Court agreed, unanimously ruling that sequences of human genes cannot be patented, while gene products can. The ruling confused both scientists and legal scholars because it didn’t provide clear reasoning for the distinction between genes and their products. The case brought to light the difficulty of dealing with these questions, which depend on technical knowledge of molecular biology, within the framework of patent law. Justice Scalia’s paragraph-long opinion in the case—in which he declared himself “unable to affirm those details on my own knowledge or even my own belief” but nonetheless concurred—may be an indication of the difficulty.
• • •
The latest controversy in this contested history, the CRISPR-Cas patent case brings out the ethical, economic, and conceptual problems with biomedical patents.
Four features of the Broad’s patent are noteworthy. First, it is sweepingly general, claiming ownership over the use of CRISPR-Cas for editing all eukaryotic DNA, which includes the DNA of all animals and plants. Second, while the Broad currently allows academics free use and grants non-exclusive licenses to companies for non-therapeutic purposes, it has granted an exclusive license to Editas Medicine, a company founded by MIT faculty member Feng Zhang, for any therapeutic applications of the technique. (The patent includes a long list of diseases, neurodegenerative to autoimmune, that might be suitable targets of CRISPR-Cas therapies.) This means that the future of CRISPR-Cas therapeutics, at least in the short term, may lie in the hands of a single startup company. Third, since the work described in the patent isn’t itself therapeutic, any company wishing to use the system as a basic research tool will need to license it. And fourth, it assigns credit for a technology derived from cumulative and collective academic research. Academics around the globe, from Japan to Lithuania to Spain and the United States, have contributed to our understanding of CRISPR-Cas. No group can claim sole credit for discovering the system or the know-how for using it to edit genomes.
No group can claim sole credit for discovering the system or the know-how for using it to edit genomes.
Understanding these features requires looking beyond patent law or universities’ economic incentives into the broader of implications of patents. Biomedical patents have been recognized to delay and impede researchers. In an effort to catch blockbuster patents that generate millions in revenue, universities are encouraged to patent everything. The resulting overhead, including material transfer agreements and reach-through licensing, strains research activities and can promote secrecy. A job market advice column in Science, for example, recommends that scientists “Patent First, Publish Second” and laments that scientists “tend to spill the beans too early and in too much detail for them to ever win a patent.” Meanwhile, most university patents aren’t even licensed, and government grants and contracts generate as much as twenty-five times more revenue for universities than patent licensing fees do. The current system also incentivizes expansive patents. As Clifton Leaf writes, the Bayh-Dole Act and the Diamond vs. Chakrabarty ruling “opened the spigots” to a rush of biomedical invention disclosures that “weren’t so much products or processes as . . . simply knowledge—or research tools to further knowledge.” The patent system has no difficulty accepting such patents. In the words of Chakrabarty’s lawyer, the ability to get work patented is “limited only by the skill of the individuals drafting the claims.”
Conventional wisdom says that without patents, companies would lack the incentive to commercialize academic ideas. This claim has been contested on multiple grounds, but even if it is true in limited cases, it is irrelevant to the CRISPR-Cas patent. CRISPR-Cas is nothing like the idealized example of a miracle drug that pharmaceutical companies must monopolize by patenting so that investors are incentivized to cover development costs. Rather, it is only one tool for doing the extensive work that may some day lead to a profitable therapeutic. Incentive to build on CRISPR-Cas has been provided by the burst of academic studies demonstrating its versatility and potential. There is no shortage of commercial interest, in spite of the patent granted to the Broad, and the arena of therapeutic applications is wide open. The expansiveness of the Broad’s patent is a barrier, not a catalyst, for companies to develop these therapies. In practice, companies backed by legal and financial muscle can compete against the Broad; others may be deterred.
Some leading academic biologists have recognized the trouble with monopolizing genome manipulation tools, but they remain in the minority.
Monopolizing a core technology developed collectively using public funding ought to require an extraordinary argument. Even if we limit ourselves to looking through the economic lens, this would require making the case that a monopoly on CRISPR-Cas therapeutics would be so wildly effective—and wide enough in scope to tackle the huge range of diseases mentioned in the patent—that it would far outweigh competitive efforts with tens, or hundreds of other companies. In the current debate, no such argument has been given.
Some leading academic biologists have recognized the trouble with monopolizing genome manipulation tools, but they remain in the minority. At an event marking the tenth anniversary of the human genome project, Nobel laureate John Sulston reiterated the danger of patenting genes and extended this critique to patents on DNA editing tools. “This is not just a philosophical point of view,” Sulston said. “It’s actually the case that monopolistic control of this kind would be bad for science, bad for consumers and bad for business, because it removes the element of competition.”
• • •
Beyond ethical and economic considerations, parceling molecular biology to private owners runs counter to the very nature of the field. Molecular biology is essentially a programming language, one for encoding information in molecules and using it to control the behavior of cells. This is why ideas from computer science have been so helpful for analyzing molecular systems. Like computer code, the molecular code is generative: it enables the creation of infinitely many constructions from finite building blocks. New systems are made by recombining those that already exist in new ways. For example, the tools that enabled researchers to import CRISPR-Cas from bacteria into eukaryotic cells—restriction enzymes—were themselves hijacked from bacteria. Granting ownership over molecular biology’s subsystems grossly restricts the creation of new systems and can result in generative gridlock.
Monopolizing a core technology developed collectively using public funding ought to require an extraordinary argument.
Economic incentives amplify the restrictions by encouraging patent claims to be as broad as possible so that competitors cannot bypass patents by making trivial modifications. Just as patenting the particular programming code that implements an algorithm would be far less valuable than patenting the algorithm itself, patenting the precise set of DNA sequences that make up one instantiation of the CRISPR-Cas system would be of little use; many insignificant spelling changes to these instructions would still enable the system to function. Biologically, it is the information, or set of instructions for the cell, that counts—and this information is inherently abstract. The Broad’s CRISPR-Cas patent is written with this knowledge in mind: it claims ownership of an abstract idea of enormous scope, not a specific invention or implementation.
The difference between a specific invention and an abstract idea is not just a theoretical distinction: it has profound consequences when ideas are parceled to different owners. The more abstract the idea, the more likely it is to be used in generating new ones.
When several ideas or tools are needed to create new ones, private ownership can hinder innovation. Legal scholars Michael Heller and Rebecca Eisenberg call this effect the “tragedy of the anticommons,” which emerges “when a user needs access to multiple patented inputs to create a single useful product.” Scientists often can’t produce these “multi-input” products because of the high overhead of sharing between private owners. Potential tools, discoveries, and lines of inquiry are obstructed by private ownership gridlock. Anticommons effects can also block humanitarian efforts, such as export of a patented drug to developing countries. Pharmaceutical companies often lack incentives to target these communities, while patents prohibit the manufacturing of cheaper generics. Groups working on behalf of these communities may not have the resources to negotiate transfer agreements, and so humanitarian applications become casualties of the anticommons.
Several damaging consequences of biomedical patents have been linked to anticommons effects. Myriad’s patents on breast cancer genes, for example, limited the availability and increased the price of breast cancer diagnostics and restricted the development of other disease diagnostics involving these genes. Patents on genes whose function was not fully understood at the time of patenting have also had detrimental effects. The CRISPR-Cas patent will likely have far more damaging ramifications since it is expected to have many more applications than the sequences of a handful of genes.
Our molecular constructions shouldn’t be thought of as inventions, but rearrangements of nature’s brilliant products. If we must award credit for inventing CRISPR-Cas, it ought to go a bacterium and the forces of evolution that have shaped it.
Yet anticommons effects are hard to quantify; they may require decades of retrospective analysis, long after damages are done. It is also inherently difficult to measure what might have come from possibilities that weren’t pursued because of anticommons gridlock. But given the conceptual similarity between software and molecular biology, much can already be learned about the effects of patents on molecular biology from software patents. The software industry boomed earlier than the biotech industry and has a faster development cycle, making it a good test case for what happens when patenting takes hold in a generative field.
In the software world, interlocking patents abound. They lead to cold wars, patent trolls, and long legal battles. Richard Stallman, a pioneer of the Free Software movement, has argued that software patenting is inherently problematic. He has pointed out that because a single piece of software may contain “thousands of ideas, it is likely to be vulnerable to hundreds of patents.” This means that “patent gridlock is ubiquitous, and the only question is whether it develops into an attack.” The result is a field in which only established giants who can bear the burden of legal uncertainty and transaction costs can participate. (This looks to be happening already with CRISPR-Cas startups.) Some have argued that these vulnerabilities are pervasive because software “represents broad classes of interactions abstractly,” making it “inherently difficult to tie down a software patent to a specific inventive concept.” But this feature isn’t unique to software. Molecular biology is susceptible to the same problems of generative gridlock.
Patent law cannot resolve these problems; they complicate any simple rule about what can be owned, such as the “product of nature” principle advocated by Eric Lander. (Such a rule depends, in any case, on our incomplete knowledge of nature's boundaries. An altered human gene, for instance, might already be produced by some naturally occurring process yet to be discovered.)
What matters in these ownership decisions isn’t what’s natural. It’s that we respect the generative nature of molecular biology and recognize that the field’s power stems from this freedom to recombine. Our molecular constructions shouldn’t be thought of as inventions, but rearrangements of nature’s brilliant products. If we must award credit for inventing CRISPR-Cas, it ought to go a bacterium and the forces of evolution that have shaped it.
• • •
A new variant of the CRISPR-Cas system, published last month by Broad scientists, showcases molecular biology’s power to recombine nature’s products. Some have pointed out that the new publication, in addition to shedding light on the biology of CRISPR-Cas, may also offer a way to skirt the patent contest with Berkeley. But the same questions of ownership and sharing plague this new system, too.
Its flaws not withstanding, the Bayh-Dole Act gave academics greater freedom to sketch the shape of the social contract between researchers and their public benefactors. Scientists should rethink the consequences of their alliance with biotech companies and corporate powers in the universities. Economic incentives are malleable and transient, but our core values aren’t. Academic researchers should not uncritically accept the demands of university intellectual property hawks who push for patents without regard for the ethical fallout or for preserving scientific openness and sharing.
We should imagine a way to distribute research products that considers the ethical and social responsibilities of scientists to the public. We can take a leaf from the software world’s book and sketch a free biology (as in “free software”).
Instead, we should imagine a way to distribute research products that considers the ethical and social responsibilities of scientists to the public. We can take a leaf from the software world’s book and sketch a free biology (as in “free software”) that respects these responsibilities. This will require new mechanisms for describing research ownership and sharing that are in the public interest and that support the university’s research branch.
In thinking about how to correct the patenting system’s violation of the commons, Yochai Benkler has proposed that research communities adopt open, “publicly minded” licensing of the sort used in the software field. This would allow academics to change the way their work may be used without having to wait for legal reforms. These commons-based licenses can also explicitly allow for humanitarian applications of research and technology, which are otherwise largely sidelined by private ownership. As Benkler noted, coordinated adoption by researchers of such mechanisms would give universities “substantial negotiating power with the biotechnology and pharmaceuticals industries.”
Synthetic biology is already making steps in this direction, with projects such as BioBricks that provide a mechanism for scientists to contribute their work to a public registry and allow others to build on it. Organizations that think about how digital information interfaces with society, such as the Electronic Frontier Foundation and the Berkman Center for Internet and Society, should partner with biologists and consider molecular information too. Building a more just sharing and distribution system for biology is possible, but it will require more academic scientists to participate in the conversation.