This essay appears in print in Thinking in a Pandemic.
Recent history tells us a lot about how epidemics unfold, how outbreaks spread, and how they are controlled. We also know a good deal about beginnings—those first cases of pneumonia in Guangdong marking the SARS outbreak of 2002–3, the earliest instances of influenza in Veracruz leading to the H1N1 influenza pandemic of 2009–10, the outbreak of hemorrhagic fever in Guinea sparking the Ebola pandemic of 2014–16. But these stories of rising action and a dramatic denouement only get us so far in coming to terms with the global crisis of COVID-19. The coronavirus pandemic has blown past many efforts at containment, snapped the reins of case detection and surveillance across the world, and saturated all inhabited continents. To understand possible endings for this epidemic, we must look elsewhere than the neat pattern of beginning and end—and reconsider what we mean by the talk of “ending” epidemics to begin with.
Historians have long been fascinated by epidemics in part because, even where they differ in details, they exhibit a typical pattern of social choreography recognizable across vast reaches of time and space. Even though the biological agents of the sixth-century Plague of Justinian, the fourteenth-century Black Death, and the early twentieth-century Manchurian Plague were almost certainly not identical, the epidemics themselves share common features that link historical actors to present experience. “As a social phenomenon,” the historian Charles Rosenberg has argued, “an epidemic has a dramaturgic form. Epidemics start at a moment in time, proceed on a stage limited in space and duration, following a plot line of increasing and revelatory tension, move to a crisis of individual and collective character, then drift towards closure.” And yet not all diseases fit so neatly into this typological structure. Rosenberg wrote these words in 1989, nearly a decade into the North American HIV/AIDS epidemic. His words rang true about the origins of that disease—thanks in part to the relentless, overzealous pursuit of its “Patient Zero”—but not so much about its end, which was, as for COVID-19, nowhere in sight.
In the case of the new coronavirus, we have now seen an initial fixation on origins give way to the question of endings. In March The Atlantic offered four possible “timelines for life returning to normal,” all of which depended the biological basis of a sufficient amount of the population developing immunity (perhaps 60 to 80 percent) to curb further spread. This confident assertion derived from models of infectious outbreaks formalized by epidemiologists such as W. H. Frost a century earlier. If the world can be defined into those susceptible (S), infected (I) and resistant (R) to a disease, and a pathogen has a reproductive number R0 (pronounced R-naught) describing how many susceptible people can be infected by a single infected person, the end of the epidemic begins when the proportion of susceptible people drops below the reciprocal, 1/R0. When that happens, one person would infect, on average, less than one other person with the disease.
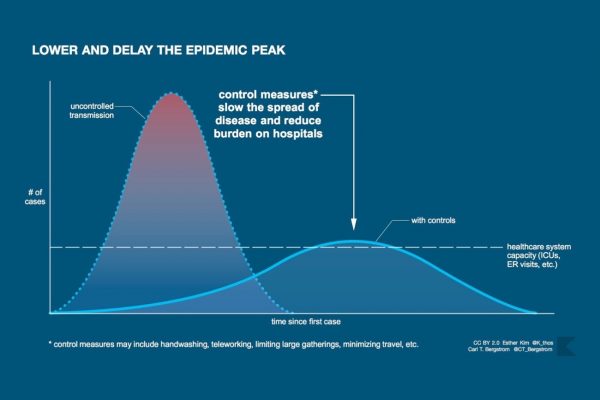
These formulas reassure us, perhaps deceptively. They conjure up a set of natural laws that give order to the cadence of calamities. The curves produced by models, which in better times belonged to the arcana of epidemiologists, are now common figures in the lives of billions of people learning to live with contractions of civil society promoted in the name of “bending,” “flattening,” or “squashing” them. At the same time, as David Jones and Stefan Helmreich recently wrote in these pages, the smooth lines of these curves are far removed from jagged realities of the day-to-day experience of an epidemic—including the sharp spikes in those “reopening” states where modelers had predicted continued decline.
In other words, epidemics are not merely biological phenomena. They are inevitably framed and shaped by our social responses to them, from beginning to end (whatever that may mean in any particular case). The questions now being asked of scientists, clinicians, mayors, governors, prime ministers, and presidents around the world is not merely “When will the biological phenomenon of this epidemic resolve?” but rather “When, if ever, will the disruption to our social life caused in the name of coronavirus come to an end?” As peak incidence nears, and in many places appears to have passed, elected officials and think tanks from opposite ends of the political spectrum provide “roadmaps” and “frameworks” for how an epidemic that has shut down economic, civic, and social life in a manner not seen globally in at least a century might eventually recede and allow resumption of a “new normal.”
These two faces of an epidemic, the biological and the social, are closely intertwined, but they are not the same. The biological epidemic can shut down daily life by sickening and killing people, but the social epidemic also shuts down daily life by overturning basic premises of sociality, economics, governance, discourse, interaction—and killing people in the process as well. There is a risk, as we know from both the Spanish influenza of 1918–19 and the more recent swine flu of 2008–9, of relaxing social responses before the biological threat has passed. But there is also a risk in misjudging a biological threat based on faulty models or bad data and in disrupting social life in such a way that the restrictions can never properly be taken back. We have seen in the case of coronavirus the two faces of the epidemic escalating on local, national, and global levels in tandem, but the biological epidemic and the social epidemic don’t necessarily recede on the same timeline.
For these sorts of reasons we must step back and reflect in detail on what we mean by ending in the first place. The history of epidemic endings has taken many forms, and only a handful of them have resulted in the elimination of a disease.
History reminds us that the interconnections between the timing of the biological and social epidemics are far from obvious. In some cases, like the yellow fever epidemics of the eighteenth century and the cholera epidemics of the nineteenth century, the dramatic symptomatology of the disease itself can make its timing easy to track. Like a bag of popcorn popping in the microwave, the tempo of visible case-events begins slowly, escalates to a frenetic peak, and then recedes, leaving a diminishing frequency of new cases that eventually are spaced far enough apart to be contained and then eliminated. In other examples, however, like the polio epidemics of the twentieth century, the disease process itself is hidden, often mild in presentation, threatens to come back, and ends not on a single day but over different timescales and in different ways for different people.
Campaigns against infectious diseases are often discussed in military terms, and one result of that metaphor is to suggest that epidemics too must have a singular endpoint. We approach the infection peak as if it were a decisive battle like Waterloo, or a diplomatic arrangement like the Armistice at Compiègne in November 1918. Yet the chronology of a single, decisive ending is not always true even for military history, of course. Just as the clear ending of a military war does not necessarily bring a close to the experience of war in everyday life, so too the resolution of the biological epidemic does not immediately undo the effects of the social epidemic. The social and economic effects of the 1918–1919 pandemic, for example, were felt long after the end of the third and putatively final wave of the virus. While the immediate economic effect on many local businesses caused by shutdowns appears to have resolved in a matter of months, the broader economic effects of the epidemic on labor-wage relations were still visible in economic surveys in 1920, again in 1921, and in several areas as far as 1930.
The history of epidemic endings has taken many forms, and only a handful of them have resulted in the elimination of a disease.
And yet, like World War One with which its history was so closely intertwined, the influenza pandemic of 1918–19 appeared at first to have a singular ending. In individual cities the epidemic often produced dramatic spikes and falls in equally rapid tempo. In Philadelphia, as John Barry notes in The Great Influenza (2004), after an explosive and deadly rise in October 1919 that peaked at 4,597 deaths in a single week, cases suddenly dropped so precipitously that the public gathering ban could be lifted before the month was over, with almost no new cases in following weeks. A phenomenon whose destructive potential was limited by material laws, “the virus burned through available fuel, then it quickly faded away.”
As Barry reminds us, however, scholars have since learned to differentiate at least three different sequences of epidemics within the broader pandemic. The first wave blazed through military installations in the spring of 1918, the second wave caused the devastating mortality spikes in the summer and fall of 1918, and the third wave began in December 1918 and lingered long through the summer of 1919. Some cities, like San Francisco, passed through the first and second waves relatively unscathed only to be devastated by the third wave. Nor was it clear to those still alive in 1919 that the pandemic was over after the third wave receded. Even as late as 1922, a bad flu season in Washington State merited a response from public health officials to enforce absolute quarantine as they had during 1918–19. It is difficult, looking back, to say exactly when this prototypical pandemic of the twentieth century was really over.
Who can tell when a pandemic has ended? Today, strictly speaking, only the World Health Organization (WHO). The Emergency Committee of the WHO is responsible for the global governance of health and international coordination of epidemic response. After the SARS coronavirus pandemic of 2002–3, this body was granted sole power to declare the beginnings and endings of Public Health Emergencies of International Concern (PHEIC). While SARS morbidity and mortality—roughly 8,000 cases and 800 deaths in 26 countries—has been dwarfed by the sheer scale of COVID-19, the pandemic’s effect on national and global economies prompted revisions to the International Health Regulations in 2005, a body of international law that had remained unchanged since 1969. This revision broadened the scope of coordinated global response from a handful of diseases to any public health event that the WHO deemed to be of international concern and shifted from a reactive response framework to a pro-active one based on real-time surveillance and detection and containment at the source rather than merely action at international borders.
This social infrastructure has important consequences, not all of them necessarily positive. Any time the WHO declares a public health event of international concern—and frequently when it chooses not to declare one—the event becomes a matter of front-page news. Since the 2005 revision, the group has been criticized both for declaring a PHEIC too hastily (as in the case of H1N1) or too late (in the case of Ebola). The WHO’s decision to declare the end of a PHEIC, by contrast, is rarely subject to the same public scrutiny. When an outbreak is no longer classified as an “extraordinary event” and no longer is seen to pose a risk at international spread, the PHEIC is considered not to be justified, leading to a withdrawal of international coordination. Once countries can grapple with the disease within their own borders, under their own national frameworks, the PHEIC is quietly de-escalated.
At their worst, epidemic endings are a form of collective amnesia, transmuting the disease that remains into merely someone else’s problem.
As the response to the 2014–16 Ebola outbreak in West Africa demonstrates, however, the act of declaring the end of a pandemic can be just as powerful as the act of declaring its beginning—in part because emergency situations can continue even after a return to “normal” has been declared. When WHO Director General Margaret Chan announced in March 2016 that the Ebola outbreak was no longer a public health event of international concern, international donors withdrew funds and care to the West African countries devastated by the outbreak, even as these struggling health systems continued to be stretched beyond their means by the needs of Ebola survivors. NGOs and virologists expressed concern that efforts to fund Ebola vaccine development would likewise fade without a sense of global urgency pushing research forward.
Part of the reason that the role of the WHO in proclaiming and terminating the state of pandemic is subject to so much scrutiny is that it can be. The WHO is the only global health body that is accountable to all governments of the world; its parliamentary World Health Assembly contains health ministers from every nation. Its authority rests not so much on its battered budget as its access to epidemic intelligence and pool of select individuals, technical experts with vast experience in epidemic response. But even though internationally sourced scientific and public health authority is key to its role in pandemic crises, WHO guidance is ultimately carried out in very different ways and on very different time scales in different countries, provinces, states, counties, and cities. One state might begin to ease up restrictions to movement and industry just as another implements more and more stringent measures. If each country’s experience of “lockdown” has already been heterogeneous, the reconnection between them after the PHEIC is ended will likely show even more variance.
So many of our hopes for the termination of the present PHEIC now lie in the promise of a COVID-19 vaccine. Yet a closer look at one of the central vaccine success stories of the twentieth century shows that technological solutions rarely offer resolution to pandemics on their own. Contrary to our expectations, vaccines are not universal technologies. They are always deployed locally, with variable resources and commitments to scientific expertise. International variations in research, development, and dissemination of effective vaccines are especially relevant in the global fight against epidemic polio.
The development of the polio vaccine is relatively well known, usually told as a story of an American tragedy and triumph. Yet while polio epidemics that swept the globe in the postwar decades did not respect national borders or the Iron Curtain, the Cold War provided context for both collaboration and antagonism. Only a few years after the licensing of Jonas Salk’s inactivated vaccine in the United States, his technique became widely used across the world, although its efficacy outside of the United States was questioned. The second, live oral vaccine developed by Albert Sabin, however, involved extensive collaboration in with Eastern European and Soviet colleagues. As the success of the Soviet polio vaccine trials marked a rare landmark of Cold War cooperation, Basil O’Connor, president of the March of Dimes movement, speaking at the Fifth International Poliomyelitis Conference in 1960, proclaimed that “in search for the truth that frees man from disease, there is no cold war.”
Yet the differential uptake of this vaccine retraced the divisions of Cold War geography. The Soviet Union, Hungary, and Czechoslovakia were the first countries in the world to begin nationwide immunization with the Sabin vaccine, soon followed by Cuba, the first country in the Western Hemisphere to eliminate the disease. By the time the Sabin vaccine was licensed in the United States in 1963, much of Eastern Europe had done away with epidemics and was largely polio-free. The successful ending of this epidemic within the communist world was immediately held up as proof of the superiority of their political system.
Western experts who trusted the Soviet vaccine trials, including the Yale virologist and WHO envoy Dorothy Horstmann, nonetheless emphasized that their results were possible because of the military-like organization of the Soviet health care system. Yet these enduring concerns that authoritarianism itself was the key tool for ending epidemics—a concern reflected in current debates over China’s heavy-handed interventions in Wuhan this year—can also be overstated. The Cold War East was united not only by authoritarianism and heavy hierarchies in state organization and society, but also by a powerful shared belief in the integration of paternal state, biomedical research, and socialized medicine. Epidemic management in these countries combined an emphasis on prevention, easily mobilized health workers, top-down organization of vaccinations, and a rhetoric of solidarity, all resting on a health care system that aimed at access to all citizens.
Still, authoritarianism as a catalyst for controlling epidemics can be singled out and pursued with long-lasting consequences. Epidemics can be harbingers of significant political changes that go well beyond their ending, significantly reshaping a new “normal” after the threat passes. Many Hungarians, for example, have watched with alarm the complete sidelining of parliament and the introduction of government by decree at the end of March this year. The end of any epidemic crisis, and thus the end of the need for the significantly increased power of Viktor Orbán, would be determined by Orbán himself. Likewise, many other states, urging the mobilization of new technologies as a solution to end epidemics, are opening the door to heightened state surveillance of their citizens. The apps and trackers now being designed to follow the movement and exposure of people in order to enable the end of epidemic lockdowns can collect data and establish mechanisms that reach well beyond the original intent. The digital afterlives of these practices raise new and unprecedented questions about when and how epidemics end.
Like infectious agents on an agar plate, epidemics colonize our social lives and force us to learn to live with them, in some way or another, for the foreseeable future.
Although we want to believe that a single technological breakthrough will end the present crisis, the application of any global health technology is always locally determined. After its dramatic successes in managing polio epidemics in the late 1950s and early 1960s, the oral poliovirus vaccine became the tool of choice for the Global Polio Eradication Initiative in the late 1980s, as it promised an end to “summer fears” globally. But since vaccines are in part technologies of trust, ending polio outbreaks depends on maintaining confidence in national and international structures through which vaccines are delivered. Wherever that often fragile trust is fractured or undermined, vaccination rates can drop to a critical level, giving way to vaccine-derived polio, which thrives in partially vaccinated populations.
In Kano, Nigeria, for example, a ban on polio vaccination between 2000 and 2004 resulted in a new national polio epidemic that soon spread to neighboring countries. As late as December 2019 polio outbreaks were still reported in fifteen African countries, including Angola and the Democratic Republic of the Congo. Nor is it clear that polio can fully be regarded as an epidemic at this point: while polio epidemics are now a thing of the past for Hungary—and the rest of Europe, the Americas, Australia, and East Asia as well—the disease is still endemic to parts of Africa and South Asia. A disease once universally epidemic is now locally endemic: this, too, is another way that epidemics end.
Indeed, many epidemics have only “ended” through widespread acceptance of a newly endemic state. Consider the global threat of HIV/AIDS. From a strictly biological perspective, the AIDS epidemic has never ended; the virus continues to spread devastation through the world, infecting 1.7 million people and claiming an estimated 770,000 lives in the year 2018 alone. But HIV is not generally described these days with the same urgency and fear that accompanied the newly defined AIDS epidemic in the early 1980s. Like coronavirus today, AIDS at that time was a rapidly spreading and unknown emerging threat, splayed across newspaper headlines and magazine covers, claiming the lives of celebrities and ordinary citizens alike. Nearly forty years later it has largely become a chronic disease endemic, at least in the Global North. Like diabetes, which claimed an estimated 4.9 million lives in 2019, HIV/AIDS became a manageable condition—if one had access to the right medications.
Those who are no longer directly threatened by the impact of the disease have a hard time continuing to attend to the urgency of an epidemic that has been rolling on for nearly four decades. Even in the first decade of the AIDS epidemic, activists in the United States fought tooth and nail to make their suffering visible in the face of both the Reagan administration’s dogged refusal to talk publicly about the AIDS crisis and the indifference of the press after the initial sensation of the newly discovered virus had become common knowledge. In this respect, the social epidemic does not necessarily end when biological transmission has ended, or even peaked, but rather when, in the attention of the general public and in the judgment of certain media and political elites who shape that attention, the disease ceases to be newsworthy.
Though we like to think of science as universal and objective, crossing borders and transcending differences, it is in fact deeply contingent upon local practices.
Polio, for its part, has not been newsworthy for a while, even as thousands around the world still live with polio with ever-decreasing access to care and support. Soon after the immediate threat of outbreaks passed, so did support for those whose lives were still bound up with the disease. For others, it became simply a background fact of life—something that happens elsewhere. The polio problem was “solved,” specialized hospitals were closed, fundraising organizations found new causes, and poster children found themselves in an increasingly challenging world. Few medical professionals are trained today in the treatment of the disease. As intimate knowledge of polio and its treatment withered away with time, people living with polio became embodied repositories of lost knowledge.
History tells us public attention is much more easily drawn to new diseases as they emerge rather than sustained over the long haul. Well before AIDS shocked the world into recognizing the devastating potential of novel epidemic diseases, a series of earlier outbreaks had already signaled the presence of emerging infectious agents. When hundreds of members of the American Legion fell ill after their annual meeting in Philadelphia in 1976, the efforts of epidemiologists from the Centers for Disease Control to explain the spread of this mysterious disease and its newly discovered bacterial agent, Legionella, occupied front-page headlines. In the years since, however, as the 1976 incident faded from memory, Legionella infections have become everyday objects of medical care, even though incidence in the U.S. has grown ninefold since 2000, tracing a line of exponential growth that looks a lot like COVID-19’s on a longer time scale. Yet few among us pause in our daily lives to consider whether we are living through the slowly ascending limb of a Legionella epidemic.
Nor do most people living in the United States stop to consider the ravages of tuberculosis as a pandemic, even though an estimated 10 million new cases of tuberculosis were reported around the globe in 2018, and an estimated 1.5 million people died from the disease. The disease seems to only receive attention in relation to newer scourges: in the late twentieth century TB coinfection became a leading cause of death in emerging HIV/AIDS pandemic, while in the past few months TB coinfection has been invoked as a rising cause of mortality in COVID-19 pandemic. Amidst these stories it is easy to miss that on its own, tuberculosis has been and continues to be the leading cause of death worldwide from a single infectious agent. And even though tuberculosis is not an active concern of middle-class Americans, it is still not a thing of the past even in this country. More than 9,000 cases of tuberculosis were reported in the United States in 2018—overwhelmingly affecting racial and ethnic minority populations—but they rarely made the news.
There will be no simple return to the way things were: whatever normal we build will be a new one—whether many of us realize it or not.
While tuberculosis is the target of concerted international disease control efforts, and occasionally eradication efforts, the time course of this affliction has been spread out so long—and so clearly demarcated in space as a problem of “other places”—that it is no longer part of the epidemic imagination of the Global North. And yet history tells a very different story. DNA lineage studies of tuberculosis now show that the spread of tuberculosis in sub-Saharan Africa and Latin America was initiated by European contact and conquest from the fifteenth century through the nineteenth. In the early decades of the twentieth century, tuberculosis epidemics accelerated throughout sub-Saharan Africa, South Asia, and Southeast Asia due to the rapid urbanization and industrialization of European colonies. Although the wave of decolonizations that swept these regions between the 1940s and the 1980s established autonomy and sovereignty for newly post-colonial nations, this movement did not send tuberculosis back to Europe.
These features of the social lives of epidemics—how they live on even when they seem, to some, to have disappeared—show them to be not just natural phenomena but also narrative ones: deeply shaped by the stories we tell about their beginnings, their middles, their ends. At their best, epidemic endings are a form of relief for the mainstream “we” that can pick up the pieces and reconstitute a normal life. At their worst, epidemic endings are a form of collective amnesia, transmuting the disease that remains into merely someone else’s problem.
What are we to conclude from these complex interactions between the social and the biological faces of epidemics, past and present? Like infectious agents on an agar plate, epidemics colonize our social lives and force us to learn to live with them, in some way or another, for the foreseeable future. Just as the postcolonial period continued to be shaped by structures established under colonial rule, so too are our post-pandemic futures indelibly shaped by what we do now. There will be no simple return to the way things were: whatever normal we build will be a new one—whether many of us realize it or not. Like the world of scientific facts after the end of a critical experiment, the world that we find after an the end of an epidemic crisis—whatever we take that to be—looks in many ways like the world that came before, but with new social truths established. How exactly these norms come into being depends a great deal on particular circumstances: current interactions among people, the instruments of social policy as well as medical and public health intervention with which we apply our efforts, and the underlying response of the material which we applied that apparatus against (in this case, the coronavirus strain SARS-CoV-2). While we cannot know now how the present epidemic will end, we can be confident that it in its wake it will leave different conceptions of normal in realms biological and social, national and international, economic and political.
Though we like to think of science as universal and objective, crossing borders and transcending differences, it is in fact deeply contingent upon local practices—including norms that are easily thrown over in an emergency, and established conventions that do not always hold up in situations of urgency. Today we see civic leaders jumping the gun in speaking of access to treatments, antibody screens, and vaccines well in advance of any scientific evidence, while relatively straightforward attempts to estimate the true number of people affected by the disease spark firestorms over the credibility of medical knowledge. Arduous work is often required to achieve scientific consensus, and when the stakes are high—especially when huge numbers of lives are at risk—heterogeneous data give way to highly variable interpretations. As data moves too quickly in some domains and too slowly in others, and sped-up time pressures are placed on all investigations the projected curve of the epidemic is transformed into an elaborate guessing game, in which different states rely on different kinds of scientific claims to sketch out wildly different timetables for ending social restrictions.
The falling action of an epidemic is perhaps best thought of as asymptotic: never disappearing, but rather fading to the point where signal is lost in the noise of the new normal—and even allowed to be forgotten.
These varied endings of the epidemic across local and national settings will only be valid insofar as they are acknowledged as such by others—especially if any reopening of trade and travel is to be achieved. In this sense, the process of establishing a new normal in global commerce will continue to be bound up in practices of international consensus. What the new normal in global health governance will look like, however, is more uncertain than ever. Long accustomed to the role of international scapegoat, the WHO Secretariat seems doomed to be accused either of working beyond its mandate or not acting fast enough. Moreover, it can easily become a target of scapegoating, as the secessionist posturing of Donald Trump demonstrates. Yet the U.S. president’s recent withdrawal from this international body is neither unprecedented nor unsurmountable. Although Trump’s voting base might not wish to be grouped together with the only other global power to secede from the WHO, after the Soviet Union’s 1949 departure from the group it ultimately brought all Eastern Bloc back to task of international health leadership in 1956. Much as the return of the Soviets to the WHO resulted in the global eradication of smallpox—the only human disease so far to have been intentionally eradicated—it is possible that some future return of the United States to the project of global health governance might also result in a more hopeful post-pandemic future.
As historians at the University of Oslo have recently noted, in epidemic periods “the present moves faster, the past seems further removed, and the future seems completely unpredictable.” How, then, are we to know when epidemics end? How does the act of looking back aid us in determining a way forward? Historians make poor futurologists, but we spend a lot of time thinking about time. And epidemics produce their own kinds of time, in both biological and social domains, disrupting our individual senses of passing days as well as conventions for collective behavior. They carry within them their own tempos and rhythms: the slow initial growth, the explosive upward limb of the outbreak, the slowing of transmission that marks the peak, plateau, and the downward limb. This falling action is perhaps best thought of as asymptotic: rarely disappearing, but rather fading to the point where signal is lost in the noise of the new normal—and even allowed to be forgotten.
Boston Review is nonprofit and relies on reader funding. To support work like this, please donate here.